tartaric acid starter core for extended release formulations
Overview of Functional Core Use in Drug Delivery
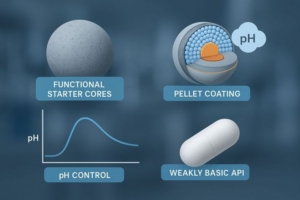
A tartaric acid starter core for extended release formulations serves as a functional nucleus around which drug layering and controlled-release coatings are built. In extended release design, dissolution and absorption of weakly basic drugs often vary with pH, which makes a stable microenvironmental pH within the dosage form critical for consistent performance. Functional cores based on tartaric acid address this problem by buffering the localized pH in the vicinity of the active pharmaceutical ingredient (API) when coated into multiparticulate dosage forms. [1]
Extended release drug products require reliable modulation of release kinetics to improve therapeutic outcomes and patient compliance. One strategy involves combining a functional core with successive layers of API and polymer coatings. The core influences the micro environmental pH, which in turn affects the solubility and dissolution rate of the drug, particularly when pH-dependent solubility limits bioavailability.
Mechanistic Rationale for tartaric acid cores
Role of Microenvironmental pH Modulation
Functional starter cores composed of tartaric acid create an acidic microenvironment within coated pellet formulations. This localized acidity benefits weakly basic drugs by enhancing solubility at elevated pH values encountered in the lower gastrointestinal tract. Unlike traditional inert cores that do not interact with the API, tartaric acid cores dissolve and release protons, lowering the surrounding pH and promoting drug release where high pH would otherwise retard dissolution. [2]
To maintain an effective micro environmental pH for the duration of drug release, the tartaric acid must not prematurely leach out of the formulation. Thus, tailored polymer film coatings are applied to the drug-layered pellets to slow tartaric acid release, ensuring that the core remains active until the API is fully released.
Influence on Release Kinetics
When tartaric acid cores are integrated into extended release systems, they influence dissolution in two complementary ways:
- Acidification within the pellet core: As dissolution medium penetrates the coatings, tartaric acid dissolves gradually, maintaining a locally acidic pH that enhances weakly basic drug solubility at sites where pH would otherwise be high (e.g., the small intestine).
- Controlled release through polymer barriers: By combining time-dependent and pH-dependent polymer layers, formulators can slow both drug and tartaric acid release. Time-dependent polymers delay the ingress of dissolution medium, while pH-dependent polymers protect the core until the desired pH trigger is reached. This dual approach prevents premature acid loss and maximizes drug exposure in target regions of the gastrointestinal tract.
Practical Considerations in Formulation
Core Properties and Selection
Functional tartaric acid cores are designed with high sphericity and controlled particle size to support uniform drug layering and reproducible coating processes. Their physical properties facilitate consistent release behavior after coating. [3]
Tartaric acid itself is a diprotic organic acid with appreciable aqueous solubility. Its acid dissociation constants (pKa values of ~2.9 and 4.4) enable it to act as a buffer in the acidic to near-neutral pH range typical of gastrointestinal environments. [4]
Integration with Coating Technologies
Formulators typically apply APIs as layers onto the tartaric acid cores using fluid bed or bottom-spray coating techniques. Successive coatings of protective and controlled-release polymers—such as time-dependent or enteric polymers—control both the release of tartaric acid and the API. [2]
The choice of polymer system influences where and when the drug is released. For example:
-
A time-dependent polymer delays exposure to dissolution medium, reducing early release in the stomach.
-
A pH-dependent polymer remains intact in acidic environments and dissolves at higher pH, triggering release at intestinal targets. [5]
Comparison with Inert Cores
Research demonstrates that formulations using tartaric acid cores show improved dissolution of weakly basic drugs at elevated pH compared to formulations with inert cores (e.g., sugar or microcrystalline cellulose). This improvement arises from the maintained acidic micro environment within the pellet, which enhances API solubility when it would typically decline at higher pH. [2]
Summary
A tartaric acid starter core for extended release formulations functions as more than a physical substrate. It actively modifies the local pH environment to enhance the dissolution of weakly basic drugs in pH conditions that would normally suppress solubility. By pairing this functional core with optimized polymer coatings, formulators can achieve controlled and targeted drug release profiles that improve performance and patient outcomes. Such systems represent an evolution beyond inert multiparticulate carriers toward interactive excipients that contribute to both dissolution kinetics and micro environmental control in oral dosage design. [1]
References
[1] N. Kállai-Szabó, et al., Pharmaceutics. 2022 Jun 18;14(6):1299. doi: 10.3390/pharmaceutics14061299
[2] K. Vlahovic, et al., Pharmaceutics 2025, 17(9), 1133; doi: 10.3390/pharmaceutics17091133
[3] https://tapellets.com/tartaric-acid-starter-pellets-as-ph-modifier/
[4] wikipedia, “tartaric acid”, link
[5] wikipedia, “enteric coating”, link




 ingredientpharm
ingredientpharm
Leave a Reply
Want to join the discussion?Feel free to contribute!