Water-insoluble weakly basic actives on tartaric acid beads – a DoE case study
Abstract
Yes, the headline is long, the overall topic not easy, and the combination of water-insoluble weakly basic actives with a DoE (Design of Experiments) method based on functional starter beads requests more than basic knowledge. With the help of a CDMO – such as Glatt Pharmaceutical Services, Binzen/Germany – the topic is immediately simplified. Let’s look over the expert’s shoulder and learn.
Weakly basic actives: Efficient development with DoE method
Water-insoluble weakly basic actives in oral formulations are tricky drug candidates for several reasons. First of all, they are water-insoluble, which is obviously not a real advantage for gaining fair drug uptake in human applications. Second, basic drugs show a fair solubility in the acidic pH range, but a weak solubility in the neutral or basic pH range. In conclusion, the better solubility is found in the human stomach, whereas a limited solubility is found in the human GI tract. Unfortunately, this is exactly the wrong order, as solubility and bioavailability in the GI tract are requested for drug uptake. A suitable drug formulation seems to be tricky. This case study will present a straight-forward DoE (Design of Experiment) method how to keep the formulation development in time and budget considering technical constraints.
Design of Experiments
The design of Experiments (DoE) method is a long-term proven method in finding optimized parameters for technical processes. For reasons of simplicity, this case study will solely focus on the experimental attempt and not describe simulation models, which – in a modern development environment – are a necessary and strong tools for increased efficiency in project realization.
Finding the right parameters
The test range for every parameter is crucial. If ranges are too narrow, the optimum of a process is not found. If ranges are too broad, the risk of failing batches is increased and the DoE cannot be evaluated. Technically, preliminary trials are requested to define feasible ranges for a DoE. Typical process parameters in production are the inlet air temperature, the process temperature, the maximum spray rate, or the atomization air pressure.
Materials
For increasing the solubility of weakly basic actives, starter pellets with an acidic character serve as drug carrier systems for modified release profile formulations and additionally serve as pH modifier. In this case, tartaric acid pellets (TAP 700) are used. The formulation foresees seal-coated and layered pellets in the size range between 800 µm and 1000 µm (Figure 1).
The drug layering liquid contains the micronized active as a water-insoluble compound. Talc is taking as antitacking agent, further a binder and an organic solvent are selected. The solid fraction in the suspension is about 20% w/w.

Figure 1: Simplified sketch of a drug layered pellet. (A) tartaric acid pellet (TAP) to improve solubility, (B) seal coat to separate acid-labile API from tartaric acid, (C) drug layer. The drug-layered pellets are finally filled in a capsule.
Response factors
Response factors are a clear measure for the goals for process development. These factors need to be individually set at the beginning and are exemplarily the yield of drug load process in percent, the process time for commercial process in hours, or the bulk volume feasible for defined capsule sizes.
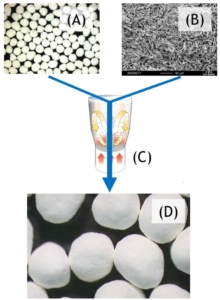
Figure 2: Scheme of a pellet layering process. Seal-coated tartaric acid pellets (A) are layered with micronized active suspended in an organic solvent (B) in a Wurster process (C). Drug-layered pellets (D) are obtained.
Processing weakly basic actives formulations
In a first process step, the seal-coated TAP 700 starter pellets are layered in a Wurster process with the micronized active powder being pre-suspended in an organic solvent. Drug-layered (DL) pellets are obtained and can further be filled in capsules (Figure 2).
Structured DoE settings and routes allow controlled change of process parameter in order to evaluate the optimum set of parameters. In this case, four parameters need to be handled as variables. The DoE plan therefore needs to be conducted in 16 steps in the stage of process development and process optimization. Fewer step numbers can be realized by excluding certain conditions through restricting the parameter space.
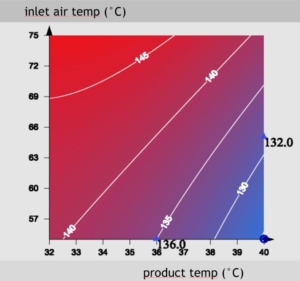
Figure 3: In this case the bulk volume is a response factor. It is influenced in a non-linear way by the inlet air temperature and the product temperature. A two-dimensional plot helps evaluating the best parameter space.
In the easiest way, a variable parameter induces direct changes to the final result. Mostly, the influence on the result is controlled by not less than two parameters and the respective parameters are not independent to each other. As an example, this is often the case for the inlet air temperature and the product temperature with respect to the bulk volume. Multi-dimensional visualizations can then be used for evaluation. Figure 3 shows the evaluation plot of a set of two exemplarily parameters.
Scale-up of weakly basic actives formulations
In further stages, such as the scale-up to pilot or scale-up to commercial phase I/II and final commercialization, the number of evaluation steps are continuously reducing, while the batch size is increasing from the kilogram to tons range (Figure 4).
So, general optimization steps towards process stability and scale-up capability need to be considered in the process optimization step. With ongoing project, multiple batch runs are performed for slight adjustments on the increasing batch size. Again, response factors are an easy measure for success. By entering the commercialization stages, the batch size does not increase anymore, and the process might be transferred to the client.

Figure 4: During process scale-up the number of evaluation steps is reducing, while the batch size is increasing. Each batch can contain up to 600 kg of product, composed of approximately 300 kg of starter pellets and more than 1000 kg of spraying suspension within a few hours, only.
Summary
This case study shows, that a tricky drug formulation of a water-insoluble weakly basic active based on tartaric acid starter pellets is one issue, which can be treated separately from process development. Formulation robustness and process stability can be easily evaluated at an early stage and iteratively optimized. Experimentally spoken, the DoE method is a strong tool for fast and structured project acceleration. By considering response factors, strict goals and targets build a strong base for up-scaling.
Acknowledgement
We acknowledge Glatt Pharmaceutical Services for a deeper insight into DoE development of oral drug formulation from small scale to commercialization. The numbers given in this case study are not representative for any formulation.

 Glatt
Glatt